The Hydrogen Fuel Cell Mass Adoption Myth: Should We Teach Hydrogen Fuel Cell Technology to Automotive Students?
Hydrogen fuel cell technology holds great promise for achieving a sustainable and zero-emission transportation system. As an alternative to internal combustion engines, hydrogen fuel cells offer several advantages, including high energy efficiency, zero tailpipe emissions, and long-range capabilities. The use of “Green Hydrogen” within the automotive field could create a world where the only emission for a vehicle is pure water.
Combining and breaking the bonds of molecules without a violent event helps to minimize electricity production effect on the environment and increase the potential of an unlimited clean power source. To allow this technology to proliferate throughout the world requires infrastructure that would be able to cleanly produce this abundant resource in a form that is easily transportable.
One of the main features of the petroleum powered vehicle is the infrastructure that is in place to allow the driver to refill in a timely manner. This change of fuel usage requires a new understanding of how to operate and service the various vehicle systems. Read on to learn more about this burgeoning technology, and to understand how to teach it to automotive students.
Principles of Hydrogen Fuel Cells
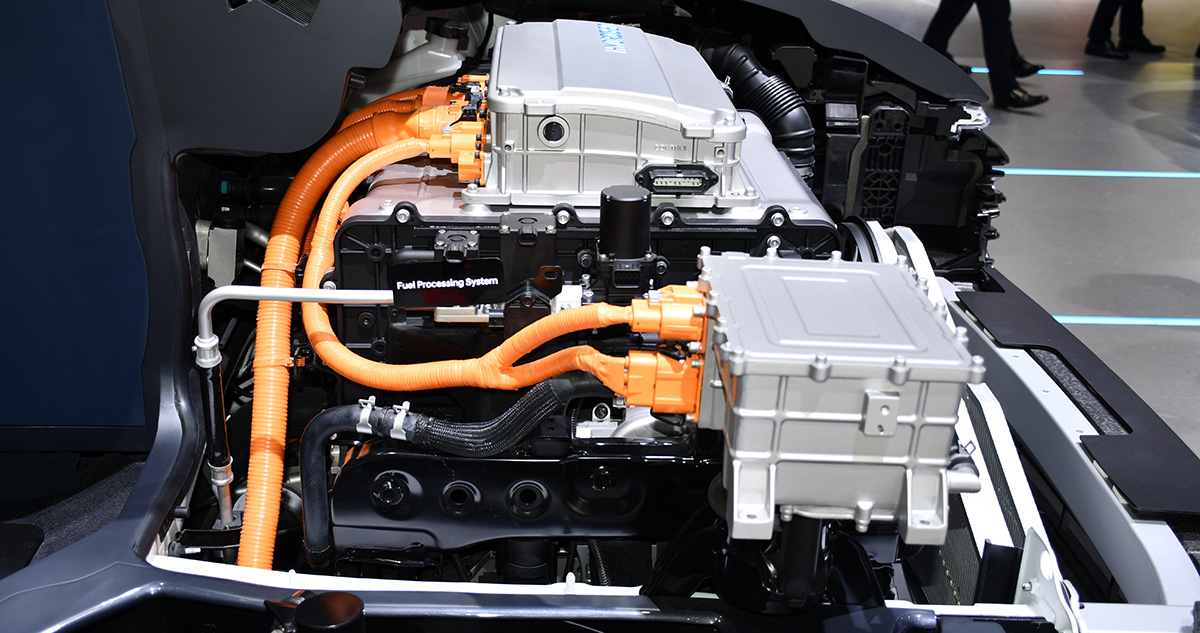
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen. Hydrogen gas (H2) is supplied to the anode side of the fuel cell, at the anode, the hydrogen molecules are split into protons (H+) and electrons (e-) through a catalyst. The protons pass through the electrolyte membrane (Proton Exchange Membrane (PEM)), while the electrons flow through an external circuit, creating an electric current. On the cathode side, oxygen from the air reacts with protons and electrons to produce water (H2O) as the only byproduct. The electrical current that is generated is a small amount per cell and not enough to provide power for any kind of electrical work.
To increase the current possibilities of the cell, multiple fuel cells are stacked together in a “fuel cell stack” which will generate a comparable amount of power.
In a Fuel Cell Electric Vehicle (FCEV) application, the power generated by the fuel cell stack is stored in a high voltage battery pack to be stored until it is required to operate the vehicle. Just like a conventional ICE powered vehicle, the fuel stack will continually produce power. Throughout the conversion process the fuel cell will become very dry which requires a moisture control which utilizes a humidifier to keep the PEM moist so the membrane will remain in operational condition throughout its life cycle. Splitting molecules to generate electricity will eventually wear out the PEM and over a period of usage the stack will have to be renewed similar to working through an ICE.
Real World Applications
Currently there are a few FCEV’s on the market. The Toyota Mirai is a hydrogen fuel cell sedan that has been in production since 2014. It offers a range of around 300 miles (483 kilometers) and emits only water vapor as a byproduct. Toyota has been at the forefront of FCEV technology development and implementation.
The Honda Clarity Fuel Cell is a hydrogen-powered sedan that offers a range of around 360 miles (579 kilometers). It has been available for lease in limited quantities in certain regions since 2016. Honda has also introduced plug-in hybrid and all-electric versions of the Clarity model; these models have been available in certain regions for a just about 10 years now in some form or another.
Hyundai has the NEXO which is an SUV powered by a hydrogen fuel cell system. It provides an estimated range of approximately 380 miles (612 kilometers) and features advanced driver assistance systems. The NEXO has been available in select markets since 2018.
The Mercedes-Benz GLC F-CELL is a hybrid vehicle that combines a hydrogen fuel cell system with a plug-in electric battery. It offers a range of approximately 300 miles (483 kilometers) from hydrogen and an additional 30 miles (48 kilometers) from the electric battery. The GLC F-CELL has been available in select markets since 2018.
Real world applications allow the OEM’s to pilot the use of the technology, along with evaluating how the driving public would want to use the vehicle. Like pure EV applications, the infrastructure needs to maintain the driving public throughout the US and the world, however we’re still in the early stages of the process.
A Note on Hydrogen
Hydrogen use in limited situations such as a dedicated route, in areas of high infrastructure saturation and situations where hydrogen fuel is generated.
Most hydrogen today is generated by mixing natural gas and steam through a process known as steam methane reformation. Utilizing a petroleum-based product to create the H2 is a self-defeating purpose as the process generates a lot of Carbon Monoxide and then H2O is added to the mixture to create more H2 and convert the output to Carbon Dioxide. If the Carbon Dioxide is not captured it can release a similar level of greenhouse gasses as burning natural gas.
Making H2 greener requires renewable energy (solar, wind and water) powering electrolyzers that separate the H2 from the Oxygen in water by passing an electrical current through the water. As the current passes through the water, H2 and Oxygen atoms are split without the production of CO2. Without this byproduct of greenhouse gas, the output is pure H2 without any harmful byproducts. This type of H2 generation can occur at any hour of the day if the green power source is producing power that is needed for the conversion process (Hydrogen Fuel Cell Partnership, n.d.).
When looking at ways to store energy with batteries or other materials, green hydrogen has a lot of promise with fewer drawbacks than other ways of generating H2. Converting H2O into H2 fuel with power generated in times when there is low demand from the electrical grid, allows the system to use the conversion process as a type of power bank. Once the ability of fuel production proliferates throughout the continent, the adoption of hydrogen powered vehicles will accelerate.
With the increased generation of H2 the price of a kilogram of H2 fuel should come down from the $10-$17 (equivalent to $5-$7.50 per gallon) to a more manageable price of $5-$10 per kilogram. Most FCEV’s utilize around 5 gallons of fuel per fill up. In comparison, pure EV’s can be charged overnight, which equates to $1 to $2 dollars a gallon.
CDX Learning Systems has developed material to help explain how a fuel cell operates in an automotive application. Understanding this conversion process is difficult for a developing technician as they cannot see the physical transition. So, we have developed different ways to consume this topic. From the learner who can read and retain the content to the learner that has to work through an animation or simulation, this multi-pronged approach makes sure the instructor can get the topic across. Light Vehicle Hybrid and Electric Vehicle Online provides access to this simulation and many others that can be utilized to allow the student to explore these diverse technological topics in an interactive way.
Related Content:- The Rise of the Solid-State Battery: Why Teaching this Tech is Becoming Increasingly Important
- 48-Volt Electrical Systems on New ICE and EV Automotive Applications: What Should Your Automotive Program Be Teaching?
- Lithium Iron Phosphate Battery Cell Chemistry is Taking Over the Automotive Market: What it Means for Auto Educators
About the Author:
Nicholas Goodnight, PhD is an ASE Master Certified Automotive and Truck Technician and an Instructor at Ivy Tech Community College. With nearly 20 years of industry experience, he brings his passion and expertise to teaching college students the workplace skills they need on the job. For the last several years, Dr. Goodnight has taught in his local community of Fort Wayne and enjoys helping others succeed in their desire to become automotive technicians.